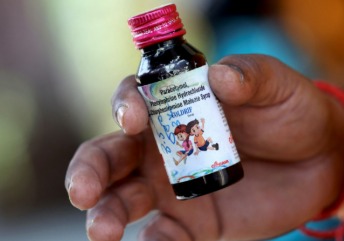
The Coldrif cough syrup tragedy has left the nation concerned about drug safety standards in India. Multiple children reportedly died after consuming the syrup, prompting investigations by health authorities and regulatory bodies.
Here are the top 5 revelations emerging from the case so far.
Top 5 Revelations
1. Contaminated Ingredients
Investigations revealed that the active ingredients in Coldrif syrup were contaminated, leading to toxic effects. Laboratory tests confirmed the presence of harmful substances beyond permissible limits.
2. Multiple Child Deaths Across States
The fatalities were not isolated. Cases have been reported in several states, highlighting widespread distribution and consumption of the syrup.
3. Lax Regulatory Oversight
Preliminary reports suggest gaps in the drug approval and monitoring system, raising questions about quality control and inspection processes in pharmaceutical manufacturing.
4. Immediate Recall Ordered
Authorities issued an urgent recall of all Coldrif syrup batches nationwide. Consumers are advised to stop using the syrup immediately and report any adverse effects to health authorities.
5. Investigations and Legal Action Underway
Police and health regulatory agencies have launched formal investigations. Legal action against the manufacturers and distributors is being considered, along with potential criminal liability for negligence.
Health Advisory
Avoid Coldrif Syrup: Do not consume the syrup until authorities confirm safety.
Seek Medical Help: Children showing unusual symptoms after consumption should receive immediate medical attention.
Report Adverse Effects: Inform local health departments or helplines about any side effects.
FAQ
Q1: What caused deaths linked to Coldrif syrup?
A1: Contaminated ingredients and toxic substances in the syrup are believed to have caused the fatalities.
Q2: How many deaths have been reported?
A2: Multiple child deaths have been reported across different states, exact numbers are being verified by authorities.
Q3: Is Coldrif syrup banned?
A3: Yes, an immediate recall has been issued by health authorities pending further investigation.
Q4: What should parents do if they have the syrup at home?
A4: Stop using it immediately, return it to the pharmacy if possible, and seek medical advice for children who consumed it.
Q5: Who is investigating the case?
A5: The case is under investigation by state police, drug regulatory authorities, and health officials.
Conclusion
The Coldrif cough syrup case underscores the critical importance of drug safety, regulatory oversight, and quality assurance in India. Families and healthcare providers are urged to remain vigilant, and authorities are working to ensure accountability and prevent further tragedies.
Published on : 15th October
Published by : SMITA
www.vizzve.com || www.vizzveservices.com
Follow us on social media: Facebook || Linkedin || Instagram
🛡 Powered by Vizzve Financial
RBI-Registered Loan Partner | 10 Lakh+ Customers | ₹600 Cr+ Disbursed
https://play.google.com/store/apps/details?id=com.vizzve_micro_seva&pcampaignid=web_share